Soil Fertility Guide
Soil pH and Salinity
Soil pH
Most Manitoba soils have a neutral (pH 7.0) to alkaline pH (pH>7.0). Soil pH influences the availability of nutrients, particularly phosphorus and micronutrients and biological activity.
Soil pH conditions result from the original soil parent material, the type of vegetation, the climate (particularly the amount of rainfall) and the age of the soils. Most agricultural soils in Manitoba are geologically young (<12,000 years), are derived from calcareous rock and developed under moderate rainfall and grassland or deciduous forest. These conditions have contributed to generally neutral to alkaline soils. The exceptions are sandy soils which have been leached or have developed under coniferous forest and peat soils.
Strong>Under low pH:
- Rhizobium bacteria which provide N fixation are inhibited
- herbicides in the imidazolinone family, such as Pursuit, break down slowly in acidic soil
Under high pH:
- availability of phosphorus and most micronutrients is reduced, making placement more important
- urea losses to volatilization are greater
- risk of injury from seed-placed urea is increased
- herbicides in the sulfonyl urea family, such as Ally and Glean and triazines (atrazine) break down slowly
Many of these fertility concerns on high pH soils are managed through timing and placement of fertilizer applications.
Management may also affect soil pH. Liming effectively raises the pH of acidic soils. Acidification of soils may occur through repeated nitrogen and sulphur application; however, on alkaline Manitoba soils this effect is negligible. Attempts to acidify alkaline soils are usually unsuccessful since the high calcium carbonate content effectively neutralizes acidity from added sulphur or nitrogen fertilizers 52 .
Efforts should be made to manage factors that increase soil pH. High pH soils may result from erosion, tillage or land leveling which removes or dilutes surface soil with more calcareous subsoil and from salt movement or salinity in the soil.
Salinity
Soil salinity is a soil condition where water soluble salts in the crop rooting zone impede crop growth. The severity of the effects and strategies to address the problem depend upon soil testing to identify the amount and type of salts present.
High salt content increases the osmotic potential of the soil solution and prevents crop uptake of water. Crops are generally most sensitive to salinity during germination and emergence. Some plants are more sensitive to salinity than others, depending on growth habit, root system, etc.
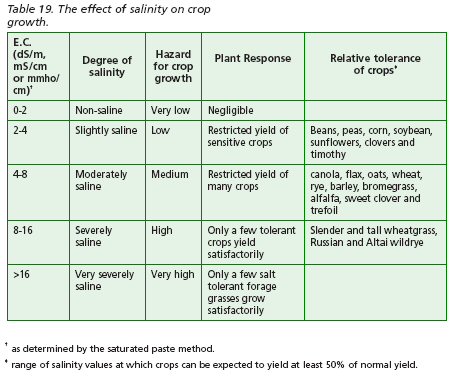
To assess the type of salinity problem, both affected and non-affected areas of the field should be sampled. Analyses should be done for electrical conductivity (E.C.), pH, cation base saturation and content of calcium, magnesium, sodium and organic matter. Electrical conductivity of a soil-water extract is an index of the concentration of dissolved salts in the soil. As salt content increases, so does the E.C. (Table 19).
Another type of soil problem occurs when sodium levels are high in relation to calcium and magnesium in the soil. These soils are very sticky and slippery when wet and very hard, cloddy and prone to crusting when dry. The sodium adsorption ratio (SAR) should be determined by the soil test lab. The SAR is the ratio of sodium to the beneficial soil structural cations, calcium and magnesium. When the SAR value exceeds 13, the soil is "sodic". If the SAR exceeds 13 and the E.C. is greater than 4, it is considered a "saline-sodic" soil.
Consult the Soil Management Guide (PDF 1.3 MB) 53 and other publications for management of saline and saline-sodic soils.
For further information, contact your MAFRD GO Representative.
